Article is a Follow-On to MolecuLight’s Receipt an Innovative Technology Contract from Vizient Last Year
PITTSBURGH, PA – (December 6, 2022) MolecuLight Corp., the leader in point-of-care fluorescence imaging for detection of wounds containing elevated bacterial loads, is featured in Vizient’s newly released Tech Watch publication as a key technology for visualizing bacterial load and its locations, and helping to reduce surgical site infections. The article, “Fluorescence Imaging: New technology enables point-of-care surgical wound bacterial assessment” is featured in Vizient’s Tech Watch (Medical Device) Volume 3 issue, issued this past week.
The Tech Watch article describes how surgical site infections (SSIs) occur in up to 38% of surgeries1 (depending on anatomical location and type of surgery) and account for 20% of all healthcare-acquired infections2. SSIs are also the costliest of these infections, extending the average length of hospital stays by 9.7 days and costing more than $20,000 per patient admission3,4.
Early and accurate diagnosis of post-surgical bacterial loads and infection is critical to enable prompt treatment before the infection worsens. Some cases require lab testing to accurately diagnose the bacteria colonizing the wound, allowing the offending bacteria to grow and spread and delay effective treatments. Test results can take days to weeks to be available and, if positive, could be too late to prevent infection.
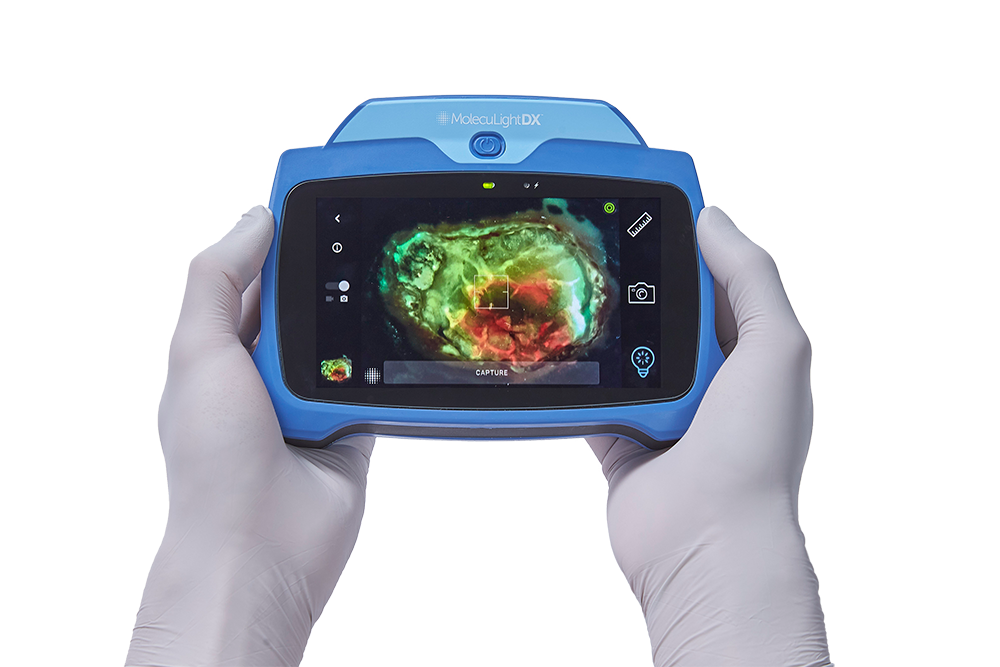
Clinicians need real-time diagnostic tools that they can use at the point-of-care to help provide immediate information on the state of the wound and possible growth of bacterial burden. The article argues that MolecuLight imaginghelps eliminate unnecessary subjectivity in assessing wounds for the presence of harmful bacteria by allowing quick and accurate visualization of locations of elevated bacteria load in wounds, along with clinical signs and symptoms. As such, it provides “invaluable real-time information to inform clinical decision-making”.
A recent per-reviewed study5 supports this position in demonstrating the benefits of using MolecuLight to help clinician visualize bacterial burden in surgical site wounds:
- 76% of surgical sites in the study that reach the stage of referral to a wound specialist had clinically significant bacterial loads (104 to 109 CFU/g), however only 6.8% exhibited symptoms of infection, resulting in delayed infection management.
- Point-of-care fluorescence imaging (using the MolecuLight i:X device) for detecting high bacterial loads improved sensitivity by 5.7-fold compared to clinical signs and symptoms alone.
- Clinician experience with fluorescence imaging and interpretation (>200 imaging sessions) increased sensitivity of fluorescence imaging to 11.3-fold higher than clinical signs and symptoms alone, and accuracy to 2.6-fold higher.
“Clinicians need an objective means of detecting infection or another surgical wound complication without having to rely on subjective judgment,” says Kylie Sandy-Hodgetts, PhD, Founder and inaugural President of the International Surgical Wound Complications Advisory Panel (ISWCAP).
“Fluorescence imaging using MolecuLight is positioned to change contemporary paradigms of post-surgical wound management due to its ability to quickly and reliably detect bacterial burden and visualize contamination at the point-of-care”.
In addition to the profile in Vizent’s Tech Watch, last year the MolecuLight i:X® fluorescence wound imaging device received an Innovative Technology contract from Vizient, Inc., the nations’ largest member-driven health care performance improvement company. The new Innovative Technology contract for MolecuLight i:X signifies to Vizient members the device’s unique qualities that potentially bring improvement to the health care industry.
“We have been working with MolecuLight since 2021 when Vizient recognized the company as an awarded supplier through our Innovative Technology Program,” said Tami Maurer, VP, Contract & Program Services at Vizient, Inc. “Carefully evaluated and selected by our member council of clinical and supply chain professionals, Vizient’s Innovative Technology Program recognizes innovative advancements in care, enabling healthcare providers to offer the highest quality care while encouraging manufacturers to continue to pioneer new solutions.”
The MolecuLight i:X and DX are the only imaging devices for the real-time detection of elevated bacterial burden in wounds that are FDA cleared and CE and Health Canada approved. With clinical evidence including over 60 peer-reviewed publications involving 1,500 patients, they are used by leading wound care facilities globally.
References
1 Alkaaki A et al. Can J Surg. 2019;62(2):111-117
2 Klevens RM et al. Public Health Rep. 2007;122(2):160-166
3 Ban KA et al. J Am Coll Surg. 2017;224(1):59-74
4 Zimlichman E et al. JAMA Intern Med. 2013;173(22):2039-2046
5 “Uncovering the high prevalence of bacterial burden in surgical site wounds with point-of-care fluorescence imaging”, Sandy Hodgetts, K. et al., Int Wound J. 2021;1–11
About MolecuLight Inc.
MolecuLight Corp. is the US subsidiary of MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight ’s suite of commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which includes two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
For more information, contact:
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
T. +1.647.362.4684
rsandler@moleculight.com
www.moleculight.com
Images:
- Attached and download at: https://moleculight.box.com/s/brze54q8e6bvwdoxtu81qdsccpcnvpa7

- Attached and download at: https://moleculight.box.com/s/tb1jnt2my59fmzjh7df5iio9n8a0nzam