Home

Transform Wound Management with Multimodal Imaging
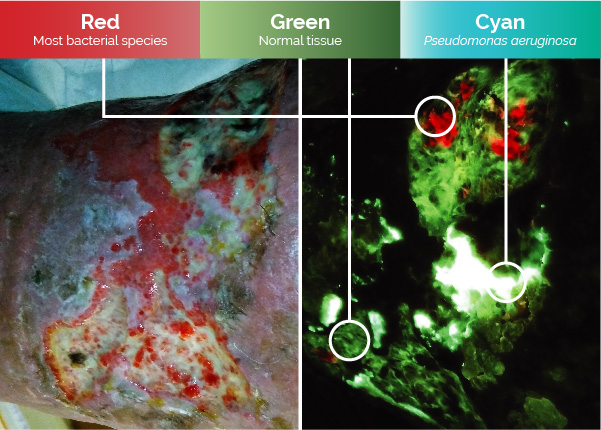
Detect
Elevated Bioburden
Locate in real-time regions containing high bacterial loads with the only non-invasive, contrast-free, FDA-cleared Class II point of care fluorescence wound imaging device.1,2
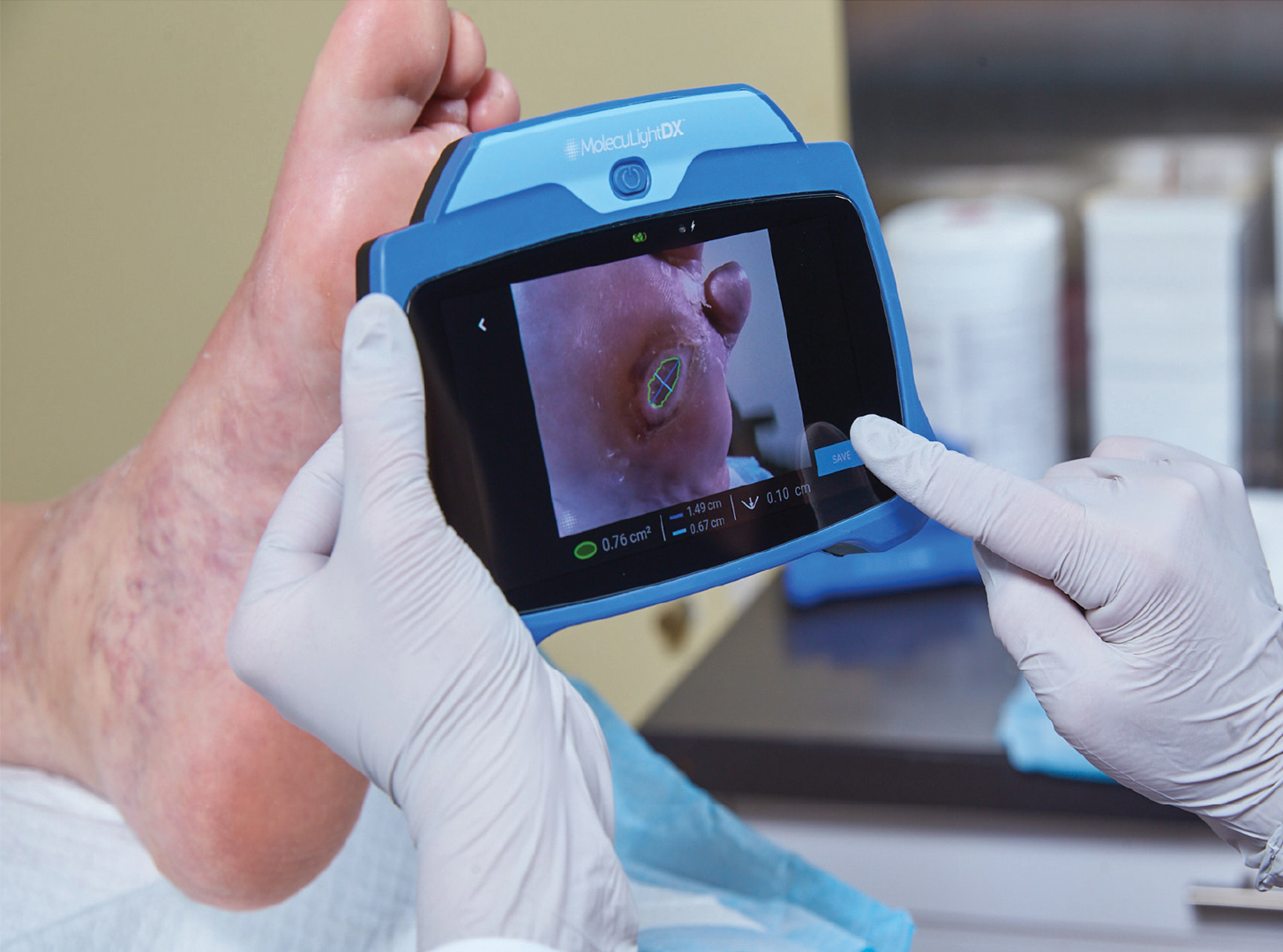
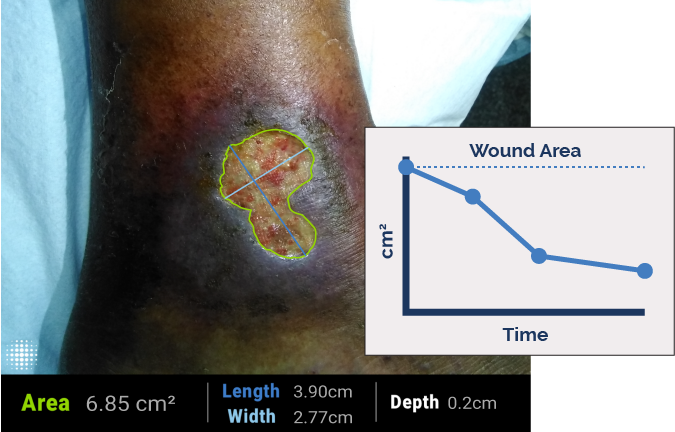
Measure
Wounds Accurately
now
available
Industry-leading validated digital wound measurement in an instant - no stickers or patient contact required. Measurements are automatically monitored and tracked over time to see wound progression.
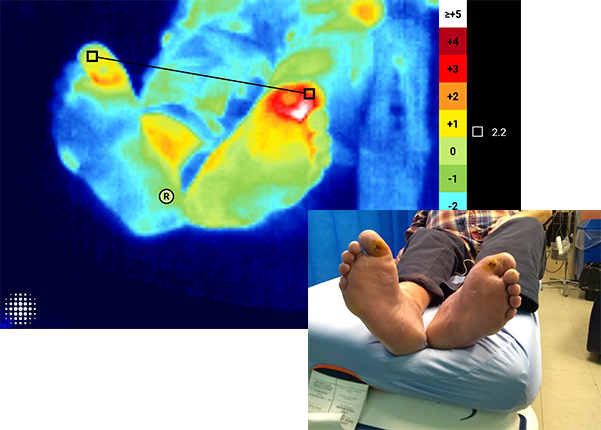
See
Thermal Changes
Quantify and document skin temperature differences with ±0.5°C accuracy. Visualize variations with a dynamic thermal map.
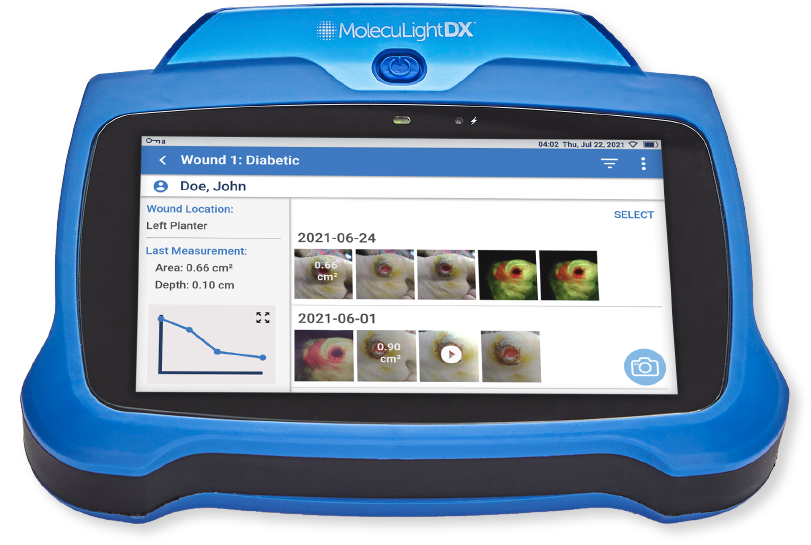
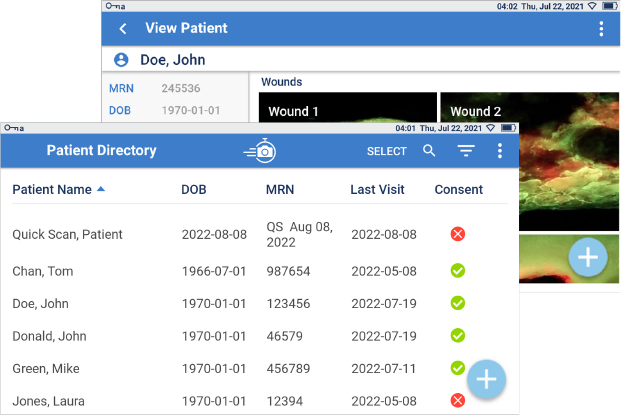
Document
With Ease
Effortless tracking and storage of wound images and details. This comprehensive evidence-based documentation helps to monitor changes in wound bioburden, treatment effectiveness and wound healing over time.
Wound Area Reduction
A prospective study showed 79% reduction in wound area by 12 weeks, when harmful bacteria detected by MolecuLight are removed.1
Wound Healing
A randomized controlled trial demonstrated 2x more wounds healed by 12 weeks using MolecuLight to inform wound care vs. Standard of Care.3
Equitable Care
In a clinical trial MolecuLight significantly improved detection of high bacterial loads in wounds across all skin tones over Standard of Care.4