Consensus on Medical Indications for Fluorescence Imaging
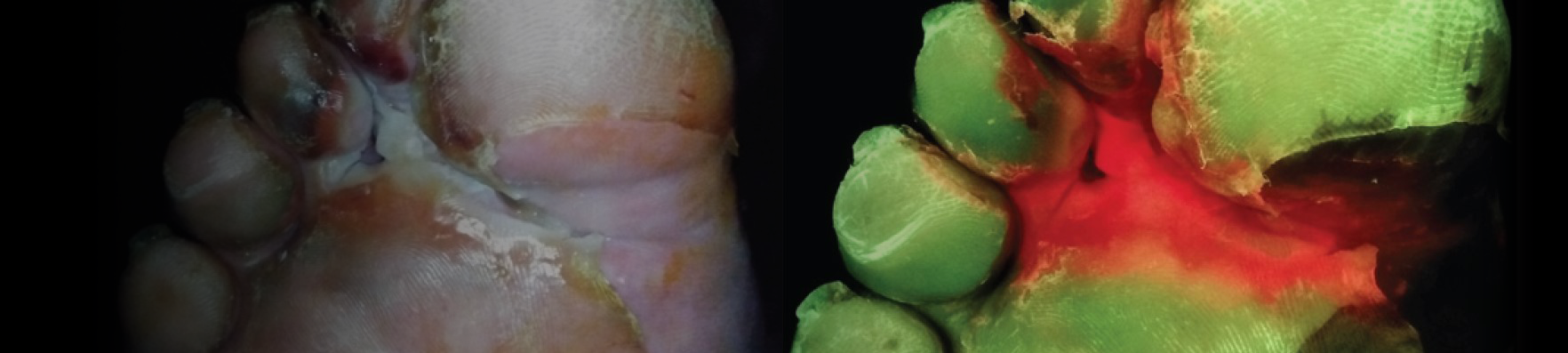
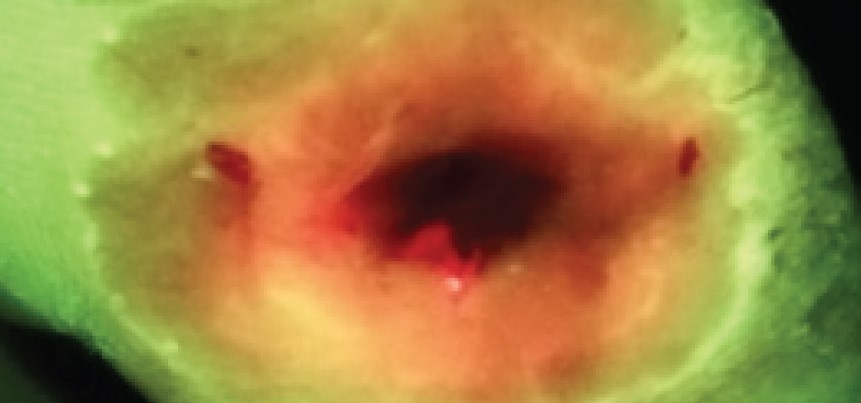
Abstract: Excessive levels of bacteria impede wound healing and can lead to infectious complications. Unfortunately, clinical signs and symptoms of elevated bacterial burden are often unreliable. As a result, point–of–care fluorescence imaging (MolecuLight), used to detect critical bacterial burden in wounds, is becoming widely recognized and adopted by clinicians across the globe as an accepted and added component of wound assessment protocol. A Delphi method was employed to establish consensus guidelines describing fluorescence imaging use. A multidisciplinary panel of 32 wound experts (56% MD, 22% podiatrist, 12.5% nurses/nurse practitioners) representing multiple sites of service (e.g., hospital outpatient, inpatient, private office, long-term care) completed two rounds of online questionnaires. The Delphi included key topics, including competencies required to perform imaging, clinical indications for imaging (e.g., signs/symptoms present, procedures warranting imaging), frequency of imaging, and a clinical workflow algorithm. Describing their clinical experiences of imaging impact, >80% reported changes in treatment plans, 96% reported that imaging-informed treatment plans led to improved wound healing, 78% reported reduced rates of amputations, and 83% reported reduced rates of microbiological sampling. The guidelines provided here will help to standardize use of fluorescence imaging among wound care providers and enhance the quality of patient care.
Payer policies will vary and should be verified prior to treatment for limitations on diagnosis, coding, or site of service requirements. The coding options listed here are commonly used codes and are not intended to be an all-inclusive list. Use of the codes provided herein allow payers to track new codes and their associated costs. The MolecuLight i:X detects wounds with elevated loads of bacteria when exposed to an excitation light. Fluorescence is only accurate under clinical darkness achieved using the DarkDrape or turning off the room lights. The MolecuLight i:X does not diagnose or treat skin wounds. The MolecuLight i:X is for prescription use only. This reimbursement guide pertains to the use of MolecuLight i:X in wound care as an imaging device.
DISCLAIMER: This reimbursement guide provides baseline reimbursement information as a convenience to MolecuLight i:X users. It is not a substitute for qualified reimbursement advice as to your specific situation. Coding, coverage and reimbursement decisions are subject to change without notice. Providers should always check with the appropriate payer before submitting claims. The use of appropriate codes, charges for service, and use of modifiers for services rendered is the responsibility of the healthcare provider. Providers are responsible for verifying coverage with payors, including the applicability of any non-coverage policies that may exist, and documentation required as justification/evidence for billed services. MolecuLight Inc. is not responsible for the timeliness, accuracy, completeness, or applicability of the information contained within this document. Since reimbursement laws, regulations, and payor policies change frequently, providers should consult their payers, coding specialists and/or legal counsel regarding coverage, coding and payment issues.
† CPT is a registered trademark of the American Medical Association
References
1 Release of 2 New Category III Codes for Real-time Fluorescence Wound Imaging to the AMA website (Updated July 1, 2020) www.ama-assn.org/system/files/2020-07/cpt-category3-codes-long-descriptors.pdf
2 AMA 2020 CPT Manual all rights reserved.
* The MolecuLight i:X and DX devices have FDA clearance for use for the detection of elevated bacterial burden in wounds when used in combination with clinical signs and symptoms