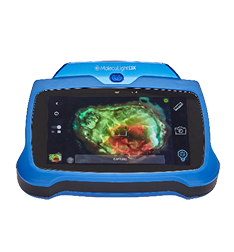
New Contract Expands VA Wound Care Center Access to MolecuLight i:X® and DX™ Point-of-Care Devices that Help Clinicians Manage Infection and Improve Outcomes
Pittsburgh, PA – January25, 2024 – MolecuLight Corp., the leader in point-of-care bacterial fluorescence imaging, announced today that the US Department of Veterans Affairs has awarded MolecuLight a Federal Supply Contract for its MolecuLight i:X® and DX™ wound imaging devices. The contract was awarded through the VA FSS (Federal Supply Schedule) program that supports the healthcare requirements of the VA and other federal government agencies. The VA FSS program manages nine multiple award Schedule programs for medical equipment, supply, pharmaceutical, and service contracts (FSC 65, 66, and 621). With over 1,700 contracts and approximately $16 billion in annual sales, the VA FSS Service provides Federal customers with access to over one million state-of-the-art commercial products and services.
“VA patients suffer from all types of wounds, including traumatic, diabetic foot and venous leg ulcers, burns and surgical site infections, to name a few, and they deserve the best treatment to help diagnose and treat their ailments, and improve their quality of life,” says Anil Amlani, MolecuLight’s CEO. “The MolecuLight suite of devices is the only Class ll FDA-cleared point-of-care platform enabling clinicians to detect harmful bacteria that is linked to infection and prevents wounds from healing1-3. Now clinicians treating Veterans can make real-time, bedside decisions to guide their treatment based on factual findingsand ensure the fastest path to healing,” Amlani continues. “MolecuLight has been providing its devices to the VA for several years, and this contract will allow wide-spread adoption and accelerated distribution of our devices across the entire VA wound care program. Our Veterans deserve the best care!”.
The MolecuLight i:X and DX are the only Class ll FDA-cleared, CE-Marked and, Health Canada approved imaging devices available in the market for the real-time detection of elevated bacterial burden in and around wounds. With clinical evidence including over 80 peer-reviewed publications involving 2,600 patients, the devices are used globally by leading wound care facilities.
About MolecuLight Corp.
MolecuLight Corp. is the US subsidiary of MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight ‘s commercial devices, which include the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, are point-of-care handheld imaging devices for the real-time detection and localization of bacterial load in wounds and digital wound measurement. MolecuLight procedures performed in the United States benefit from an available reimbursement pathway which include two CPT® codes for physician work to perform “fluorescence imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment. The company is also commercializing its unique fluorescence imaging platform technology for other global markets with relevant unmet needs in food safety, consumer cosmetics and other key industrial markets.
References
1Xu L, McLennan SV, Lo L, et al. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care. 2007;30(2):378-380.
2Rahim K, Saleha S, Zhu X, Huo L, Basit A, Franco OL. Bacterial Contribution in Chronicity of Wounds. Microb Ecol. 2017;73(3):710-721.
2Armstrong DG, Edmonds ME, Serena TE. Point-of-care fluorescence imaging reveals extent of bacterial load in diabetic foot ulcers. Int Wound J. Feb 2023;20(2):554-566.
For more information, contact:
PR & Media Relations
MolecuLight Inc.
T. +1.647.362.4684
info@moleculight.com
www.moleculight.com