Back to ALL Research Publications
![]()
![]()
![]()
![]()
![]()
 PUBLICATION_
PUBLICATION_ 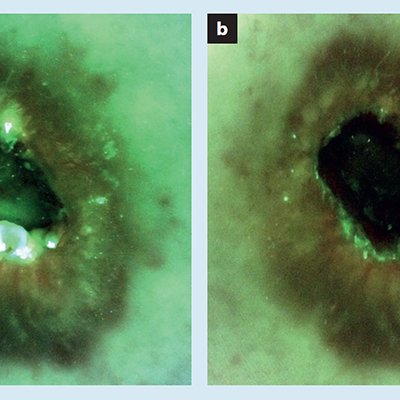 PUBLICATION_
PUBLICATION_ 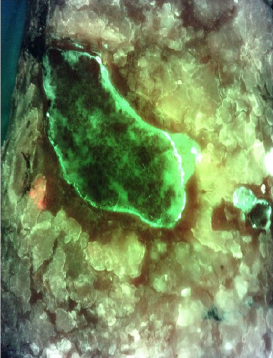 PUBLICATION_
PUBLICATION_ 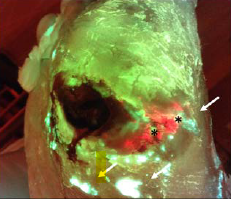 PUBLICATION_
PUBLICATION_
Research Category: ,
The Role of Autoflorescence Imaging Device in the Evaluation of Bacteria Burden Control
Related Publications
Related Material
 PUBLICATION_
PUBLICATION_ Bacterial fluorescence imaging as a predictor of skin graft integration in burn wounds
 PUBLICATION_
PUBLICATION_ A novel debridement device for the treatment of hard-to-heal wounds: a prospective trial
 PUBLICATION_
PUBLICATION_ Fluorescence-Based Evaluation of Bacterial Load in Perilesional Skin: A Comparison Between Short Stretch Bandage and Zinc Oxide Bandage
 PUBLICATION_
PUBLICATION_ A prospective multi-site observational study incorporating bacterial fluorescence information into the UPPER/LOWER wound infection checklists
MolecuLight Headquarters
425 University Avenue Suite 700 Toronto, ON M5G 1T6 Canada
US Address MolecuLight Corp. 2403 Sidney Street, Suite 286 Pittsburgh, PA 15203
T. 1-877-818-4360
North American Toll Free:
1-877-818-4360
F.+1 647-362-4730
E: info@moleculight.com
©2026
The MolecuLight® i:X and MolecuLightDX™ Imaging Devices are approved by Health Canada for sale in Canada and has CE marking for sale in the European Union.
The MolecuLight™ i:X and DX Imaging Devices have received FDA clearance.










