MolecuLight Platform is Becoming the Standard-of-Care for Real-Time Imaging of Elevated Bacterial Burden in Wounds Across All Wound Care Settings
TORONTO, CANADA – (October 14, 2021) MolecuLight Inc., the leader in point-of-care fluorescence imaging for real-time detection of wounds containing elevated bacterial loads, announced the launch of the MolecuLightDX™, a new point-of-care device model targeted at the unique needs of new expanding wound care market segments in the USA. The DX is an expansion of MolecuLight’s product line and compliments the MolecuLight i:X®, the “workhorse” wound imaging device that has quickly become a standard in wound care practices worldwide, with over 2,000 units sold.
“The i:X and DX are the only commercially-available point-of-care devices to enable real-time detection of elevated bacterial burden in wounds. With the introduction of the MolecuLightDX, we are thrilled to expand our product line and provide added functionality for these wound care market segments,” says Anil Amlani, MolecuLight’s CEO.
Specifically, the MolecuLightDX has the following new features frequently required in these segments:
- Comprehensive EMR (electronic medical record) integration options for multiple EMR environments,
- Patient-centric user interface and workflow to allow for easy patient and wound tracking,
- An Administrator workflow and system configuration capability, and
- Docking system for easier charging of the devive.
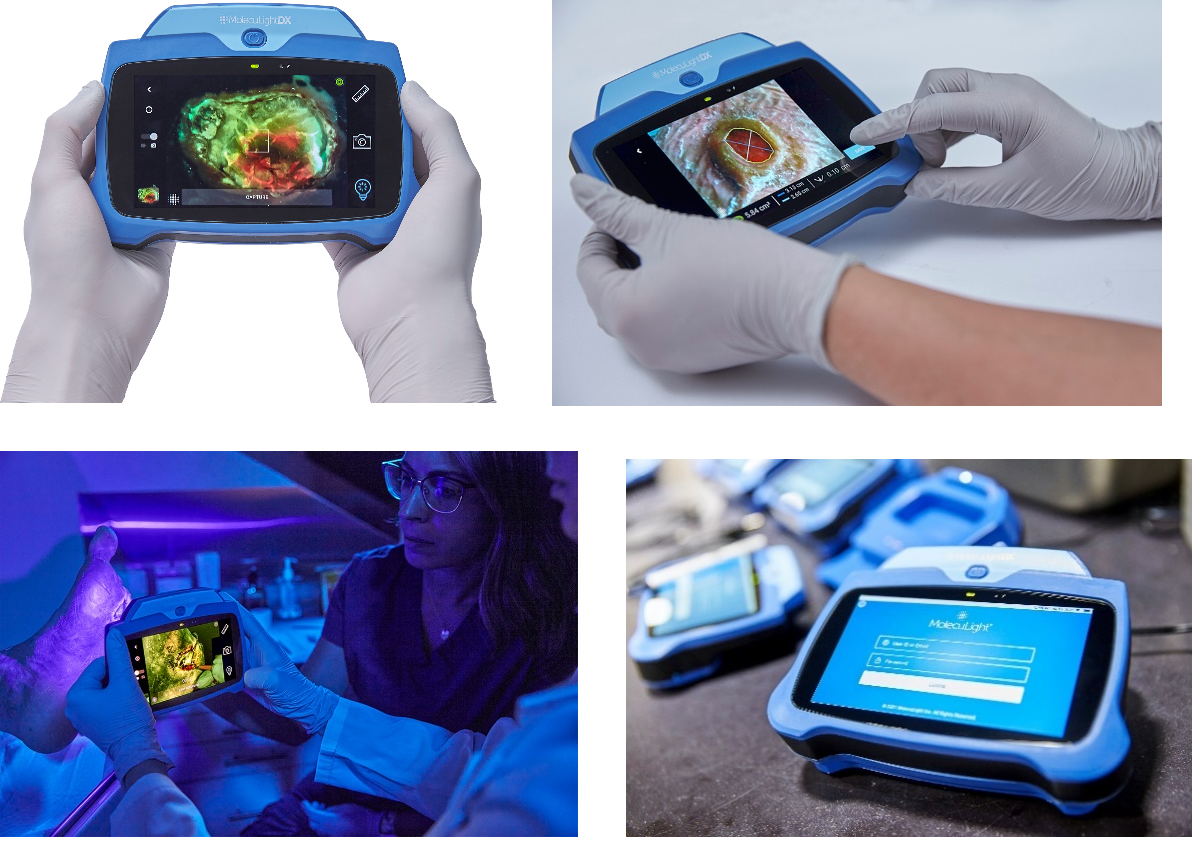
As with the i:X, the DX has the same accurate, rapid digital wound measurement for documentation of procedures and of wound progression. Newly available on the DX is a stickerless measurement capability which automatically measures wound area without the need for wound stickers.
“With the expansion of our product line, we can now offer clinicians in any care setting the unmatched capabilities of the MolecuLight platform, with a feature set and price point that matches their specific needs”, says Amlani. “MolecuLight customers will continue to receive our comprehensive activation and training support on both platforms, including on-site training with patients, e-Learning courses and certification and ongoing in-person and remote support by our Clinical Applications team.”
In addition, all MolecuLight procedures will be able to benefit from the reimbursement pathway available in the United States for the MolecuLight procedure, which is applicable to both the MolecuLight i:X andDX devices. The reimbursement pathway includes two CPT® codes for physician work to perform “fluorescence wound imaging for bacterial presence, location, and load” and facility payment for Hospital Outpatient Department (HOPD) and Ambulatory Surgical Center (ASC) settings through an Ambulatory Payment Classification (APC) assignment.
The MolecuLightDX has received FDA clearance for sale in the USA, as well as the CE Mark and Health Canada approval for commercial availability in Europe and Canada.
The MolecuLightDX will be displayed in the MolecuLight exhibit booth at the upcoming clinical conference, SAWC (Symposium on Advanced Wound Care) Fall 2021, on Sunday, October 31, 2021 at 9:00 am at Caesars Palace in Las Vegas, Nevada. To request an on-site clinical demonstration of the MolecuLightDX, please go to www.moleculight.com, or email info@moleculight.com.
About MolecuLight Inc.
MolecuLight Inc., a privately-owned medical imaging company that has developed and is commercializing its proprietary fluorescent imaging platform technology in multiple clinical markets. MolecuLight’s suite of commercially released devices, including the MolecuLight i:X® and DX™ fluorescence imaging systems and their accessories, provide point-of-care handheld imaging devices for the global wound care market for the real-time detection of wounds containing elevated bacterial burden (when used with clinical signs and symptoms) and for digital wound measurement. The company is also commercializing its unique fluorescence imaging platform technology for other markets with globally relevant, unmet needs including food safety, consumer cosmetics and other key industrial markets.
Rob Sandler
Chief Marketing Officer
MolecuLight Inc.
M. +1.647.362.4684
rsandler@moleculight.com
www.moleculight.com
Download for Images:
- Hands holding the MolecuLightDX
- MolecuLightDX with new Stickerless digital wound area measurement
- MolecuLightDX in clinical setting
- MolecuLightDX unit in docking station