Back to ALL Research Publications
![]()
![]()
![]()
![]()
![]()
 PUBLICATION_
PUBLICATION_ 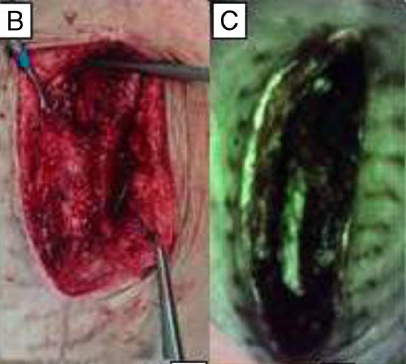 PUBLICATION_
PUBLICATION_ 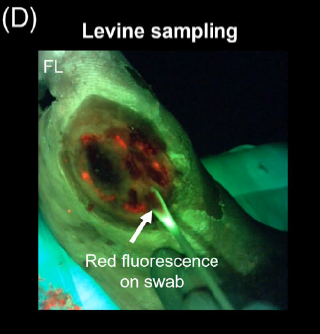 PUBLICATION_
PUBLICATION_
Research Category: ,
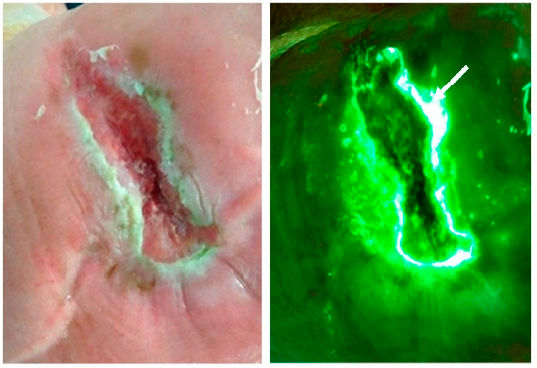
Assessing Pediatric Burn Wound Infection Using a Point-of-Care Fluorescence Imaging Device
Related Publications
Related Material
 PUBLICATION_
PUBLICATION_ Routine fluorescence imaging to detect wound bacteria reduces antibiotic use and antimicrobial dressing expenditure while improving healing rates: Retrospective analysis of 229 foot ulcers
 PUBLICATION_
PUBLICATION_ Multidisciplinary Strategies With Real-Time Fluorescence Images and Negative Pressure Wound Therapy to Manage Organ/Space Surgical Site Infection in Transplanted Kidneys
 PUBLICATION_
PUBLICATION_ Lights, fluorescence, action—Influencing wound treatment plans including debridement of bacteria and biofilms
MolecuLight Headquarters
425 University Avenue Suite 700 Toronto, ON M5G 1T6 Canada
US Address MolecuLight Corp. 2403 Sidney Street, Suite 286 Pittsburgh, PA 15203
T. 1-877-818-4360
North American Toll Free:
1-877-818-4360
F.+1 647-362-4730
E: info@moleculight.com
©2026
The MolecuLight® i:X and MolecuLightDX™ Imaging Devices are approved by Health Canada for sale in Canada and has CE marking for sale in the European Union.
The MolecuLight™ i:X and DX Imaging Devices have received FDA clearance.










