Home
Clinical Guidelines for the Management of Elevated Bacterial Burden in Wounds

Assessment
Quantitative Bacterial load is considered to be the best indicator of wound infection, and may cause chronic inflammation that can inhibit wound healing2,23,10.
Cleaning
According to clinical guidelines, wound cleansing involves removal of contaminants including bacteria from the wound7,8.
Debridement
Clinical guidelines for the treatment of venous ulcers indicate debridement is required to remove excessive bacterial burden from the wound10.
Sampling
Clinical guidelines recommend determining bacterial load of a pressure ulcer by biopsy or quantitative swab10.
Documentation
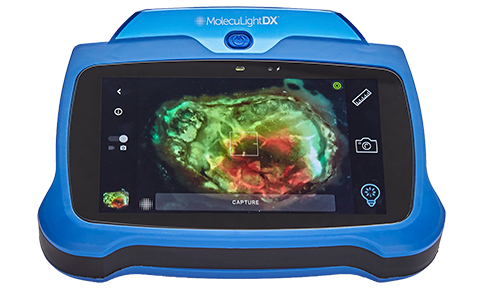
The MolecuLight i:X and DX allow clinicians to document the wound by saving the digital wound area measurement as well as saving the image of the wound with a fluorescence image for detection of elevated bacterial load (in combination with clinical signs and symptoms).
Treatment Selection
Clinical guidelines recommend considering wound bacterial burden in any decisions regarding topical antibiotics, systemic antibiotics, or surgical debridement19,20.
Antibiotic Stewardship
Clinical guidelines that wound bacterial load should be considered when making decisions regarding discontinuing topical antimicrobials to minimize the possibility of resistance16.
Patient Engagement
A critical part of managing hard-to-heal, and indeed healing wounds and any other medical condition, is the patient following the agreed care path21.
2 Heather L. Orsted et al., Foundations of Best Practice for Skin and Wound Management Skin: Anatomy, Physiology and Wound Healing
3 Robson MC et al., Guidelines for the treatment of venous ulcers. Wound Repair Regen. 2006 Nov-Dec;14(6):649-62
4 Emily Haesler et al., National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline, 2014
5 O’Donnell TF et al., Society for Vascular Surgery; American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery, J Vasc Surg. 2014 Aug;60(2 Suppl):3S-59S
6 American Diabetes Society Diagnosis and Management of Diabetic Foot Infections, 2020
7 Emily Haesler et al., National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline, 2014
8 Connie L. Harris et al., Best Practice Recommendations for the Prevention and Management of Surgical Wound Complications
10 Martin C. Robson et al., Guidelines for the treatment of venous ulcers
11 O’Donnell TF Jr et al., Society for Vascular Surgery; American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2014 Aug;60(2Suppl):3S-59S
12 Ousey K. et al., International Consensus Document: Identifying and treating foot ulcers in patients with diabetes: saving feet, legs and lives. J Wound Care 2018; 27
13, 19, 23 Emily Haesler et al., National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline, 2014
14 Hopf HW et al., Guidelines for the treatment of arterial insufficiency ulcers. Wound Repair Regen. 2006 Nov-Dec;14(6):693-710
15, 16, 20 Whitney J, et al., Guidelines for the treatment of pressure ulcers. Wound Repair Regen. 2006 Nov-Dec;14(6):663-79
21 Atkin L. et al., Implementing TIMERS: the race against hard-to-heal wounds. J Wound Care 2019; 28(3 Suppl 3):S1–S49
Watch MolecuLight i:X in action
The MolecuLight i:X is a point-of-care imaging device for performing digital wound measurement and to help clinicians identifying more wounds with elevated bacterial load (>104 CFU/g) than standard clinical examination alone.
Intended Use & Indication for Use
The MolecuLight i:X and DX are intended for use as handheld imaging tools that allow clinicians diagnosing and treating skin wounds, at the point of care, to:
(i) View and digitally record images of a wound,
(ii) Measure and digitally record the size of a wound, and
(iii) View and digitally record images of fluorescence emitted from a wound when exposed to an excitation light.
The fluorescence image, when used in combination with clinical signs and symptoms, has been shown to increase the likelihood that clinicians can identify wounds containing bacterial loads >104 CFU per gram as compared to examination of clinical signs and symptoms alone.
• The MolecuLight i:X and DX devices should not be used to rule-out the presence of bacteria in a wound.
• The MolecuLight i:X and DX devices do not diagnose or treat skin wounds.


New peer-reviewed publications featuring MolecuLight appeared in:
 i:X
i:X
 DX
DX

























